Loading...
|
Trivitron Healthcare’s broad expertise in manufacturing allows it to concentrate on the niche market segments of healthcare. It not only caters to the inaccessible geographical locations, but also manufactures medical devices belonging to specialties, which are in its embryonic stages. With 9 manufacturing facilities, Trivitron is now an indisputable partner in the emerging healthcare industry producing a wide range of equipment, laboratory reagents, diagnostic kits and even protective apparel for radiology departments in hospitals across the globe.
Trivitron's manufacturing dreams became a reality when the company established the Trivitron Medical Technology Park (TMTP) in Irungattukottai, near Chennai. A 25-acre of lush green field now serves as the manufacturing paradise by housing Labsystems Diagnostics IVD Factory, BioSystems Trivitron Diagnostics Ltd. and Aloka Trivitron Medical Technologies.

Aloka Trivitron Medical Technologies facility focuses on electronic imaging devices, such as heart monitors, Ultrasound machines and Color Doppler technologies adhering to ISO 13485 and CE certification.
Extending its wings, the facility takes on everything right from raw material sourcing and assembling to testing and packaging of Ultrasound machines and Color Doppler technologies. This CE/JGMP certified ATMT manufacturing facility exports over 1000 Ultrasound Machines every year.

Soon after making a name for itself among Pathology laboratories within India, Trivitron established a joint venture with Biosystems SA, Spain. Biosystems, which has its manufacturing facility in Barcelona, established Biosystems Trivitron Diagnostics Ltd. inside the Trivitron Medical Technology Park (TMTP).
This is their first manufacturing facility established outside Spain for the Clinical Chemistry Products. Designed in keeping with the international standards including ISO, GMP-DCA to make and market clinical diagnostic products, this joint venture facility manufactures and exports a wide range of Biochemistry Reagents.

Anchoring the 'Make in India' program, Trivitron launched the South Asia's first Labsystems Diagnostics IVD Factory, an Indo-Finnish joint collaboration at the Trivitron Medical Technology Park (TMTP). Through the IVD factory, Trivitron offers laboratory chemicals and reagents to empower every neighbourhood pathology laboratory to diagnose a wide variety of diseases and perform tests for different ailments.
With this establishment, Trivitron brought the Finnish Technology from its subsidiary Labsystems Diagnostics Oy. This CE certified manufacturing facility in Chennai manufactures and exports a wide range of IVD products for Biochemistry, Haematology, Newborn Screening Kits, Molecular Diagnostics and Point-of-care.

Trivitron’s OT manufacturing facility located in Chennai helms as a designing and manufacturing facility for mobile & modular healthcare solutions and medical support systems. These systems are researched, designed, developed and manufactured based on a flexible installation approach, enabled by the company's innovative and patented end products.
This CE certified Trivitron facility manufactures and exports Operating Room products like Guided Air Flow OTs, Laminar Flow OTs, Ultraclean Modular OTs, Pendants, Bed Head Units and many other turn-key concepts.
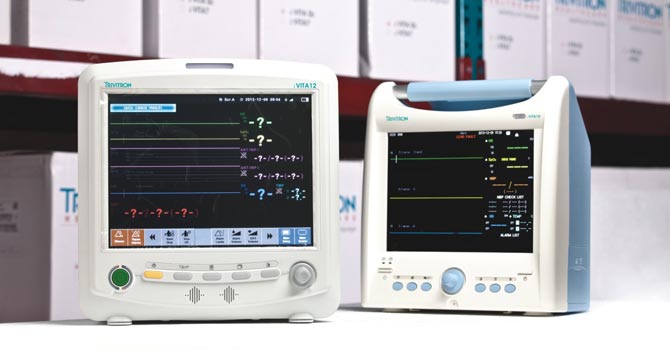
Trivitron’s yet another facility in Chennai manufactures and markets advanced technology cardiac diagnostic instruments. Products manufactured here include, ECG recording systems, Stress Test Systems, Holter ECGs, ABPM record units, Patient Monitoring systems, ICU Ventilators Patient Monitors, Defibrillators and IT based telemedicine. This CE certified manufacturing facility is one of the largest facilities in Asia catering to all the emerging markets and to the world eventually.

Trivitron has joined hands with Kiran Medical Systems, a global leader in Image Enhancement and Radiation Protection Products to extend its Imaging and Radiology portfolio. This CE/US FDA certified facility of Kiran Medical Systems located in Mumbai manufactures and exports X-ray Accessories and Radiation Protection Apparels to over 165 countries. Spreading over an area of 70,000 sq.ft. the products are in line with the international standards of ISO, DIN, ANSI, JS, BS, ISI and others.
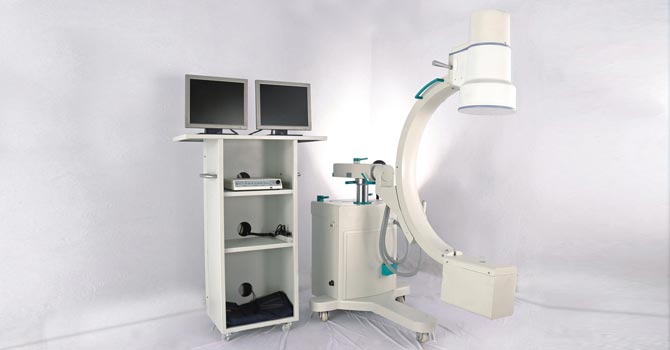
Ever since its inception, Vision Medicad stands as a benchmark provider of CE certified X-Ray machines. With a notion to empower Vision Medicad, Trivitron empowered the organization by modernizing and expanding two of its facilities in Pune to manufacture X-Ray and C-Arm.
Vision’s AERB approved manufacturing facility at Pune manufactures and exports imaging products like X-ray, C-Arm, etc along with Portable Cathlab. Vision’s products are being marketed under Trivitron’s Pride Series banner to emerging global markets in South Asia, South East Asia, Middle East and Africa.

Labsystems Diagnostics facility in Finland manufactures and exports CE certified Newborn Screening and Diagnostics kits for Gastro and Cardiac Panel, Infectious Diseases and Women's Health product lines. These Finnish technology products are being sold in North Europe, South Europe, Middle East and North Africa, South Asia, Southeast Asia, China and Latin America, as well as through various strategic partners' and sales and distribution networks in North America. Labsystems Diagnostics serves as the European arm of Trivitron Healthcare in Laboratory Diagnostics, POC and Home Tests.

Trivitron has acquired 60 per cent stake in Bome Sanayi Urunleri Dis. Tic. Ltd. Sti of Turkey, which mainly makes In-Vitro diagnostic devices. With this acquisition, Trivitron now has 9 production facilities located in India, Finland and Turkey and will have direct access to the Turkish, Middle Eastern, African, South and South East Asian Markets. Now it will also produce IVD diagnostics kits using Finnish (fluorescent/ mass spectrometry) and Turkish (Elisa) techniques in Finland, Turkey and India.